Guidelines for using
Blue Light Cystoscopy with Cysview®
Blue Light Cystoscopy (BLC®) with Cysview is included in Canada, US, and international industry guidelines.
Blue Light Cystoscopy (BLC®) with Cysview is included in Canada, US, and international industry guidelines.
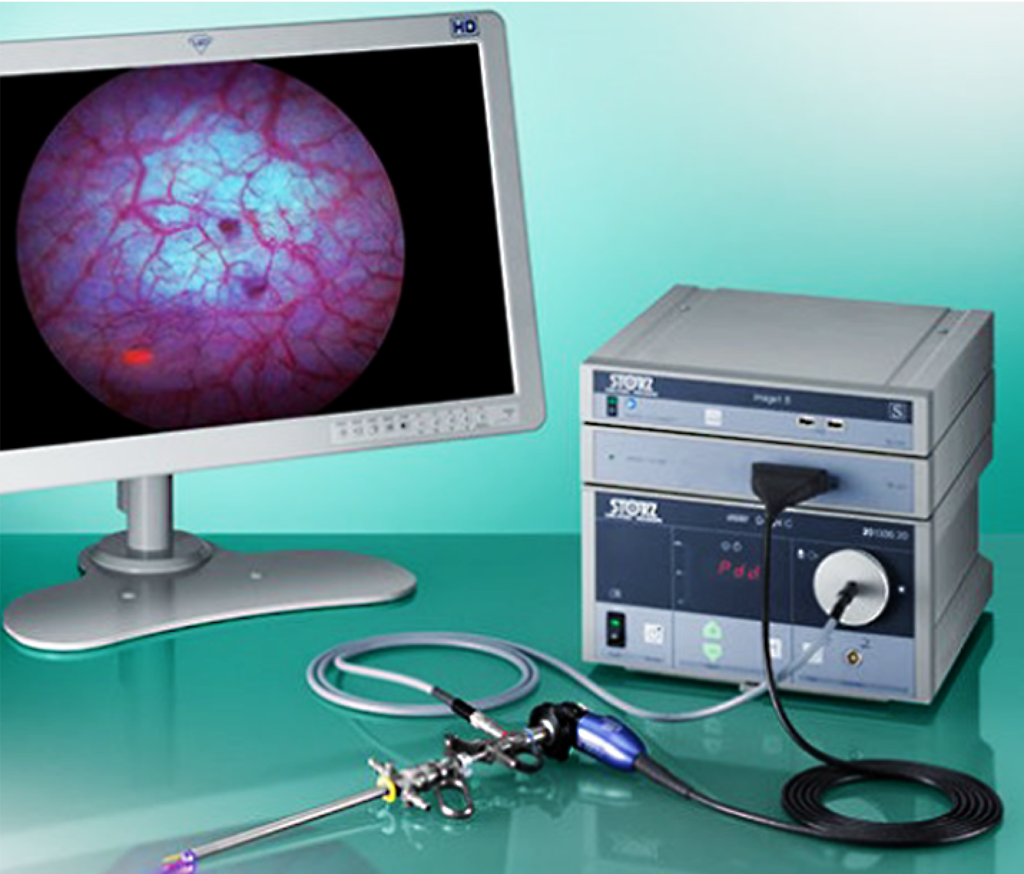
KARL STORZ was the first to provide BLC equipment in Canada. The IMAGE1 S™ Saphira™ system includes a camera-control-unit with camera head and resectoscope with a special light source that supports use of both white and blue light.
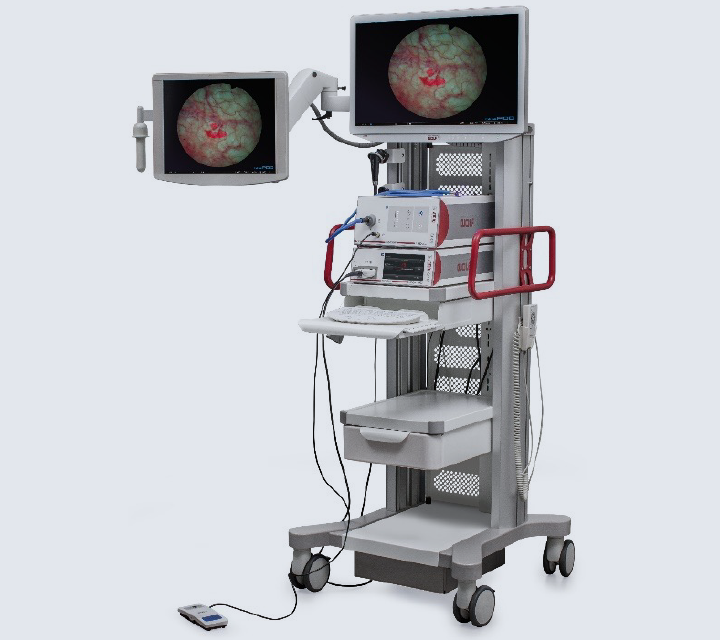
RICHARD WOLF launched its System Blue equipment in 2023. Visualization includes white light mode, bluePDD SIM mode, and bluePDD Color Contrast SIM mode. The camera head generated 4K UHD resolution.
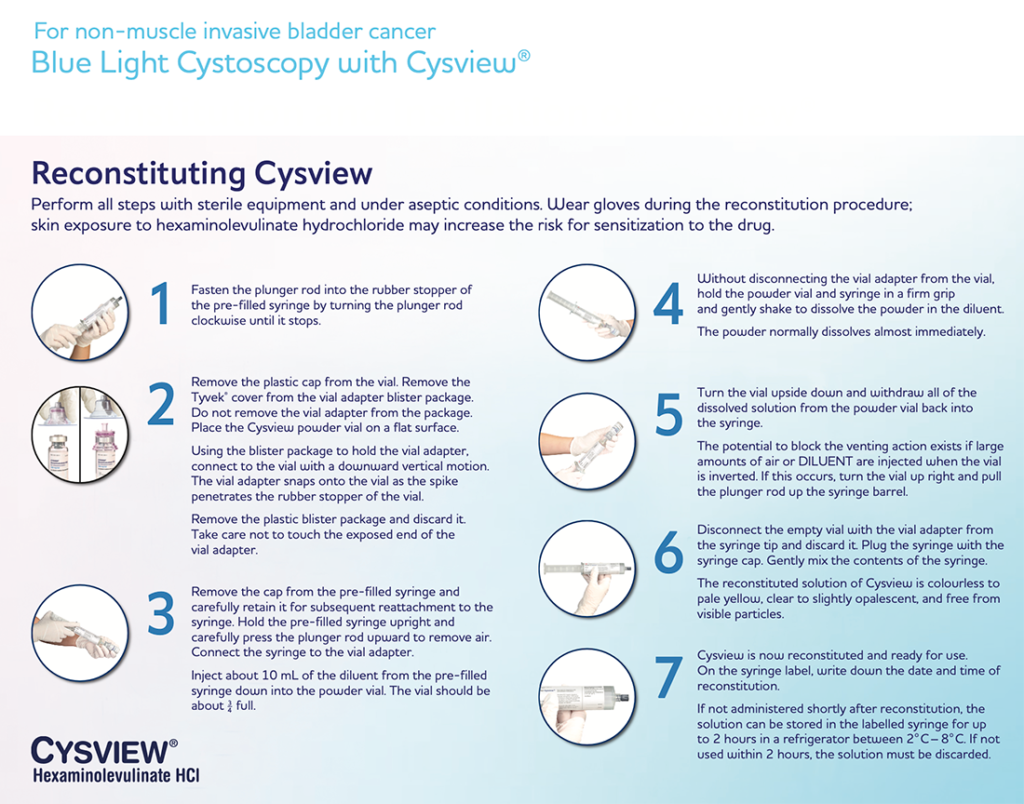
A Step-By-Step Guide to the Reconstitution and Instillation of Cysview Using the Pre-filled Syringe
This video walks you through the steps of
Cysview reconstitution and instillation.
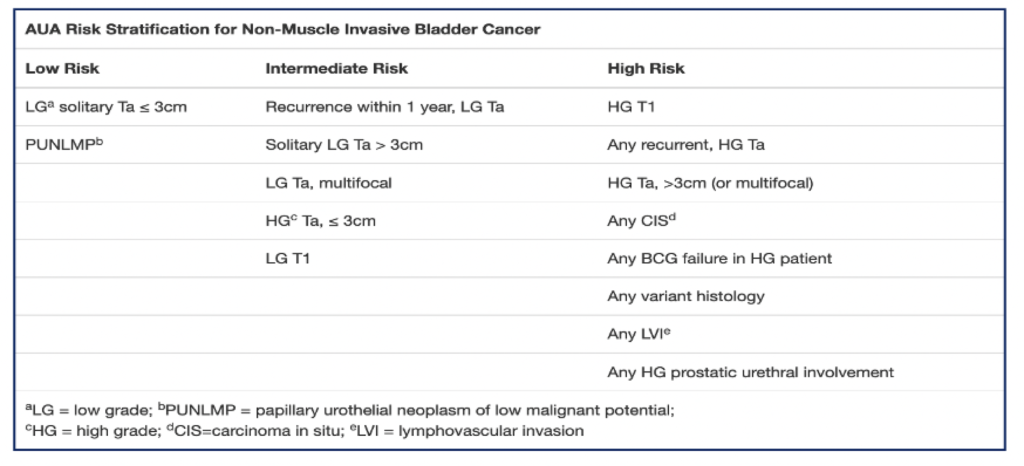
Use Blue Light Cystoscopy (BLC®) with Cysview in patients with non-muscle invasive bladder cancer (NMIBC).
Use in first TURBT and the management of intermediate and high-risk patients.
In a patient with NMIBC, a clinician should offer BLC with Cysview, if available, to increase detection and decrease recurrence. (Moderate Recommendation; Evidence Strength: Grade B).1
In a prospective, comparative, within-patient controlled, multicenter phase III study in the detection of Ta/T1 tumors in patients who had previously undergone a cystoscopy and had suspicion of or confirmed NMIBC: Out of 286 patients with at least one Ta or T1 tumor, 16% were detected only with BLC with Cysview (p=0.001).2
Strong recommendation to use BLC with Cysview.3
BLC with Cysview is strongly recommended in intermediate and high-risk patients.
The decision to use BLC with Cysview in this instance should be made on a patient-by-patient basis by taking into account the benefit from accurately diagnosing more clinically significant disease cases versus the risk of false positives. The incidence of false positives decreases as time from BCG therapy increases.4
This scenario is an important endpoint for BLC with Cysview in high-risk patients.3
For intermediate-risk patients
For high-risk patients
A majority of the panel (13/17) thought that BLC with Cysview would be of benefit before initiating intravesical therapy in patients at intermediate risk or high risk of recurrence, based on the patient’s not having undergone a previous TURBT using BLC with Cysview.3
In patients with a positive cytology and negative WLC, a clinician should consider prostatic urethral biopsies and upper tract imaging, as well as enhanced cystoscopic techniques (BLC, when available), ureteroscopy, or random biopsies. (Expert Opinion).1

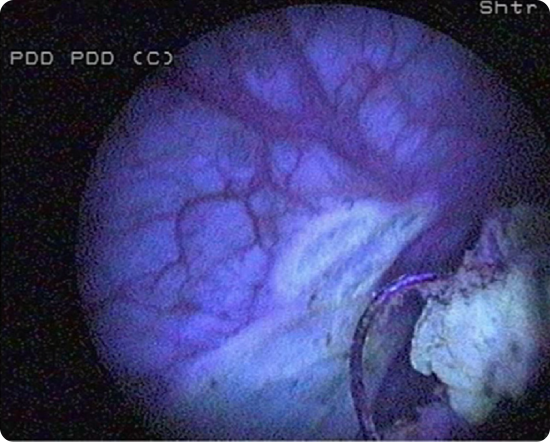
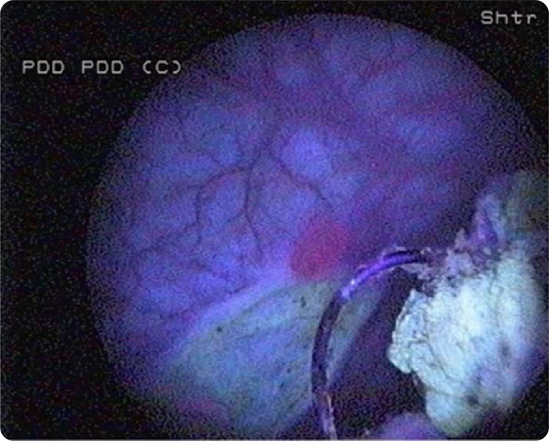
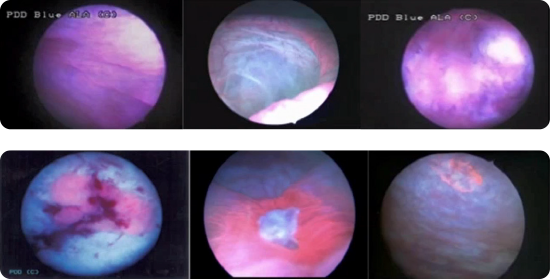
Ensure adequate time after instillation of Cysview
If you’d like more information about how and when to use Cysview, trouble-shooting tips, or anything else, request a visit from your Photocure Key Account Manager. Contact Us
Cysview is indicated as an adjunct to White Light Cystoscopy in the diagnosis and follow up of non-muscle invasive bladder cancer, including carcinoma in situ (CIS), in patients with known or suspected bladder cancer to increase tumor detection.
Only approved cystoscopic equipment should be used, equipped with necessary filters to allow both White Light Cystoscopy (WLC) and Blue Light (wavelength 360–450nm) fluorescence Cystoscopy (BLC®). Training in Blue Light Cystoscopy with an approved Photodynamic Diagnosis (PDD) System is essential prior to the use of Cysview.
Limitations of Use: Cysview is not a replacement for random bladder biopsies or other procedures used in the detection of bladder cancer.
Contraindications: Cysview is contraindicated in patients with porphyria and/or hypersensitivity to the active substance or to any ingredient in the formulation or component of the container. False-positive fluorescence may result from tangential light, scope trauma from a previous cystoscopic examination, and/or bladder inflammation particularly from intravesical Bacillus Calmette–Guérin (BCG) or chemotherapy treatments. No specific drug interaction studies have been performed.
Warnings and Precautions: Do not use in patients with gross hematuria. Do not use in patients at high risk of bladder inflammation, e.g., less than 90 days after intravesical BCG or chemotherapy. Do not use for retrograde uretero-renoscopy. Cysview has not been studied in pregnant women or pediatric populations. Cysview may not detect all malignant lesions. Very rare instances of hypersensitivity, including anaphylactic shock, have been reported during post-marketing use of Cysview. Advance life support facilities should be readily available. Biopsy/resect bladder mucosal lesions only following completion of both White Light and Blue Light Cystoscopy.
Adverse Reactions: Most of the reported adverse reactions in clinical studies were transient and mild or moderate in intensity: bladder spasm 2.0%; dysuria 1.6%; bladder pain 1.4%; and hematuria 1.0%. The adverse reactions observed were expected based on previous experience with standard cystoscopy and TURBT procedures.
BLC® and Cysview®
are registered trademarks of Photocure ASA.
©2024 Photocure Canada/Cysview. All rights reserved.
Product Monograph | Privacy Policy | Terms of Use
Information on this website is intended for Canada audiences only.
If you are from the USA, please visit Cysview.com
