CLINICAL EVIDENCE
Blue Light Cystoscopy with Cysview (BLC) significantly improves the detection of papillary non-muscle invasive bladder cancer (NMIBC) compared to White Light Cystoscopy (WLC) alone.1
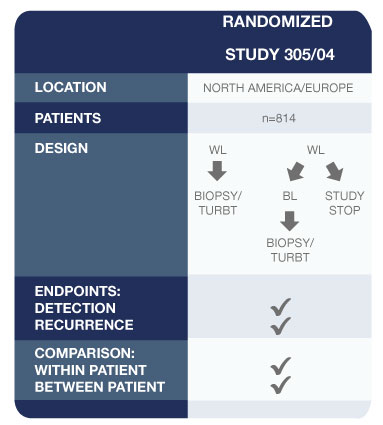
Pivotal Study for Approval of Blue Light Cystoscopy with Cysview®2
This study demonstrated that Cysview results in a statistically significant improvement in the detection of Ta/T1 tumors.1
Improved detection of tumours can result in more appropriate treatment due to better visualization of the tumours leading to more complete resection, benefitting the patient.1
- Other findings of this study:
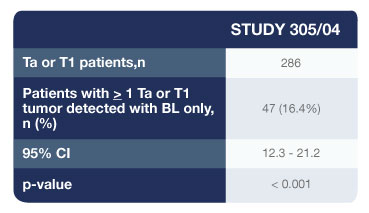
- 286 patients were confirmed to have Ta or T1 bladder cancer on biopsy
- 47 patients (16.4%) had one or more Ta or T1 tumours (p=0.001) that were found only with Cysview
- Many of the additional tumours detected by BLC were clinically significant, including grades 2 & 3 and T1 tumours that had been missed with WLC
- The investigators found that "Cysview-induced fluorescence cystoscopy significantly improves the detection of Ta and T1 lesions”.
- The study conclusions were that:
- BLC with Cysview “significantly improves the detection of bladder cancer leading to a more complete resection and significantly better disease-free survival.”
- “These results are in line with those of previous studies demonstrating improved detection of lesions with Cysview.”
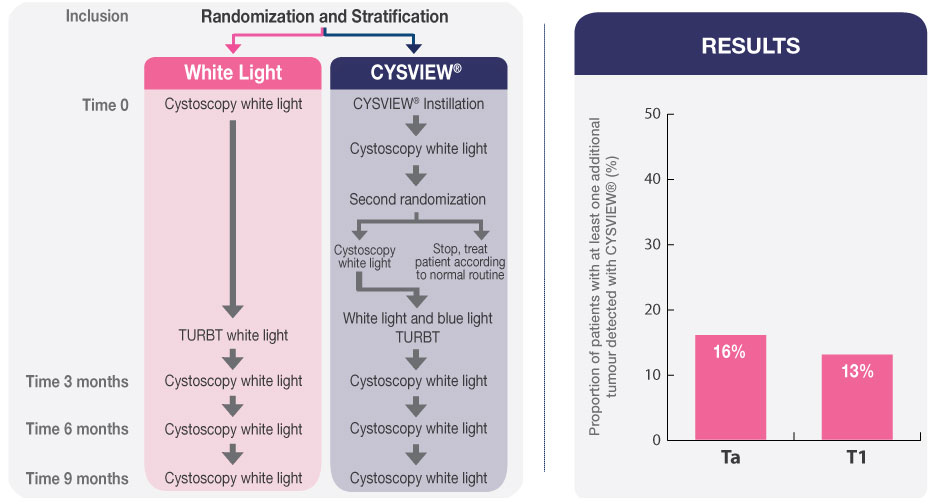
The study results show that WLC alone can miss some tumours and leave residual tumour following resection in the bladder. Compared to WLC alone, Blue Light Cystoscopy with Cysview enhances the visibility of Ta/T1 NMIBC tumours, which can result in improved detection and more complete resection.
Indication for Cysview® (hexaminolevulinate HCl)
Cysview is indicated as an adjunct to White Light Cystoscopy in the detection of non-muscle invasive papillary bladder cancer in patients with known or suspected bladder cancer. Only approved cystoscopic equipment should be used, equipped with necessary filters to allow both White Light Cystoscopy (WLC) and Blue Light (wavelength 360–450nm) fluorescence Cystoscopy (BLC®). Training in Blue Light Cystoscopy with an approved Photodynamic Diagnosis (PDD) System is essential prior to the use of Cysview.Important Risk & Safety Information
Limitations of Use Cysview is not a replacement for random bladder biopsies or other procedures used in the detection of bladder cancer.
Contraindications Cysview is contraindicated in patients with porphyria and/or hypersensitivity to the active substance or to any ingredient in the formulation or component of the container. False-positive fluorescence may result from tangential light, scope trauma from a previous cystoscopic examination, and/or bladder inflammation particularly from intravesical Bacillus Calmette–Guérin (BCG) or chemotherapy treatments. No specific drug interaction studies have been performed.
Warnings and Precautions Do not use in patients with gross hematuria. Do not use in patients at high risk of bladder inflammation, e.g., less than 90 days after intravesical BCG or chemotherapy. Do not use for retrograde uretero-renoscopy. Cysview has not been studied in pregnant women or pediatric populations. Cysview may not detect all malignant lesions. Very rare instances of hypersensitivity, including anaphylactic shock, have been reported during post-marketing use of Cysview. Advance life support facilities should be readily available. Biopsy/resect bladder mucosal lesions only following completion of both White Light and Blue Light Cystoscopy.
Adverse Reactions Most of the reported adverse reactions in clinical studies were transient and mild or moderate in intensity: bladder spasm 2.4%; dysuria 1.8%; bladder pain 1.7%; and hematuria 1.7%. The adverse reactions observed were expected based on previous experience with standard cystoscopy and TURBT procedures.
For additional information about Cysview, please refer to the product monograph.
Note: Cysview (hexaminolevulinate (HAL) HCl) is used with Blue Light Cystoscopy (BLC) as an adjunct to White Light Cystoscopy (WLC) and not separately. Cysview with BLC may be referred to in a number of ways, such as Cysview, Cysview with BLC, Cysview with Photodynamic Diagnosis (PDD) System, HAL with BLC and HAL with PDD.
- Cysview® Canada Product Monograph, Jan 11, 2022.
- Hermann GG, Mogensen K, Carlsson S, et al. Fluorescence-Guided Transurethral Resection of Bladder Tumours Reduces Bladder Tumour Recurrence Due to Less Residual Tumour Tissue in Ta/T1 Patients: A Randomized Two-Centre Study. BJU Int. 2011;108(8b):E297-E303.

 contact
contact